The drug is usually very well tolerated.
In clinical and post-marketing (*) studies of the drug Diflucan®, the following adverse reactions were noted.
From the nervous system: often – headache; infrequently – dizziness *, convulsions *, change in taste *, paresthesia, insomnia, drowsiness; rarely – tremor.
From the digestive system: often – abdominal pain, diarrhea, nausea, vomiting *; infrequently – flatulence, dyspepsia *, dryness of the oral mucosa, constipation.
From the hepatobiliary system: often – increased serum activity of aminotransferases (ALT and AST), alkaline phosphatase; infrequently – cholestasis, jaundice *, increased concentration of bilirubin; rarely – hepatotoxicity, in some cases fatal, liver dysfunction *, hepatitis *, hepatocellular necrosis *, hepatocellular damage.
On the part of the skin: often – rash; infrequently – itching, urticaria, increased sweating, drug rash (including persistent drug rash); rarely – exfoliative skin lesions *, including Stevens-Johnson syndrome and toxic epidermal necrolysis, acute generalized exanthematous pustulosis, facial edema, alopecia *; frequency unknown – drug rash with eosinophilia and systemic symptoms (DRESS syndrome).
From the hematopoietic system *: rarely – leukopenia, including neutropenia and agranulocytosis, thrombocytopenia, anemia.
From the immune system *: anaphylaxis (including angioedema).
From the side of the cardiovascular system *: rarely – an increase in the QT interval on the ECG, ventricular tachysystolic arrhythmia of the “pirouette” type (torsade de pointes).
From the side of metabolism *: rarely – an increase in the concentration of cholesterol and triglycerides in blood plasma, hypokalemia.
From the musculoskeletal system: infrequently – myalgia.
Others: infrequently – weakness, asthenia, increased fatigue, fever, vertigo.
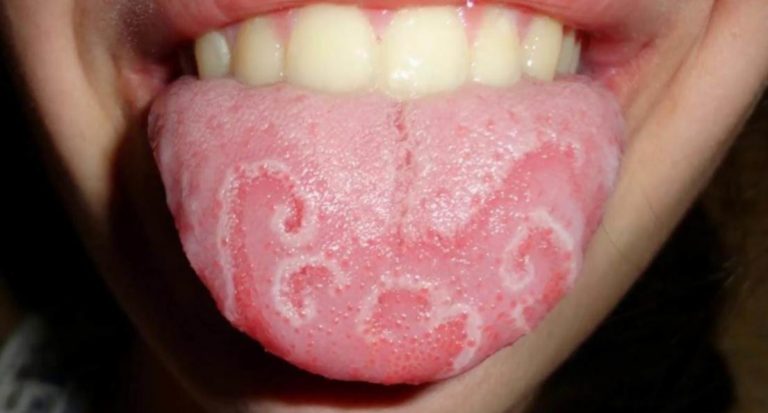
In some patients, especially those with serious diseases such as AIDS or cancer, during treatment with Diflucan® and similar drugs, changes in blood counts, kidney and liver function were observed, but the clinical significance of these changes and their relationship with treatment have not been established.
Contraindications for use
simultaneous use of terfenadine during repeated use of fluconazole at a dose of 400 mg / day or more;
simultaneous use with drugs that increase the QT interval and are metabolized by the CYP3A4 isoenzyme, such as cisapride, astemizole, erythromycin, pimozide and quinidine;
lactase deficiency, galactose intolerance, glucose-galactose malabsorption;
children under 3 years of age (for this dosage form);
hypersensitivity to fluconazole, other components of the drug, or azole substances with a structure similar to fluconazole.
With care: liver failure; renal failure; the appearance of a rash against the background of the use of fluconazole in patients with superficial fungal infection and invasive / systemic fungal infections; simultaneous use of terfenadine and fluconazole at a dose of less than 400 mg / day; potentially proarrhythmic conditions in patients with multiple risk factors (organic heart disease, electrolyte imbalance and concomitant therapy that promotes the development of such disorders).
how long do fluconazole side effects last
diflucan side effects anxiety
fluconazole and alcohol
fluconazole interactions
fluconazole reviews
fluconazole contraindications
fluconazole uses
when to take second fluconazole